Welcome
Stakeholders dialogue
This initiative was launched after a workshop with stakeholders, primarily European and national CLP authorities, in September 2009, on possible ways of fulfilling the new CLP requirements (see workshop proceedings).
In 2011 and in 2012 respectively, A.I.S.E., in conjunction with National Associations, shared the first and second edition of its stakeholder briefing document with Member States experts to provide an update on developments since the September 2009 Workshop and organised information exchange with them.
For more information on DetNet access to authorities, see DetNet structure.
A.I.S.E. is open to feedback from Member State competent authorities and enforcement agencies (contact us here).
DetNet structure
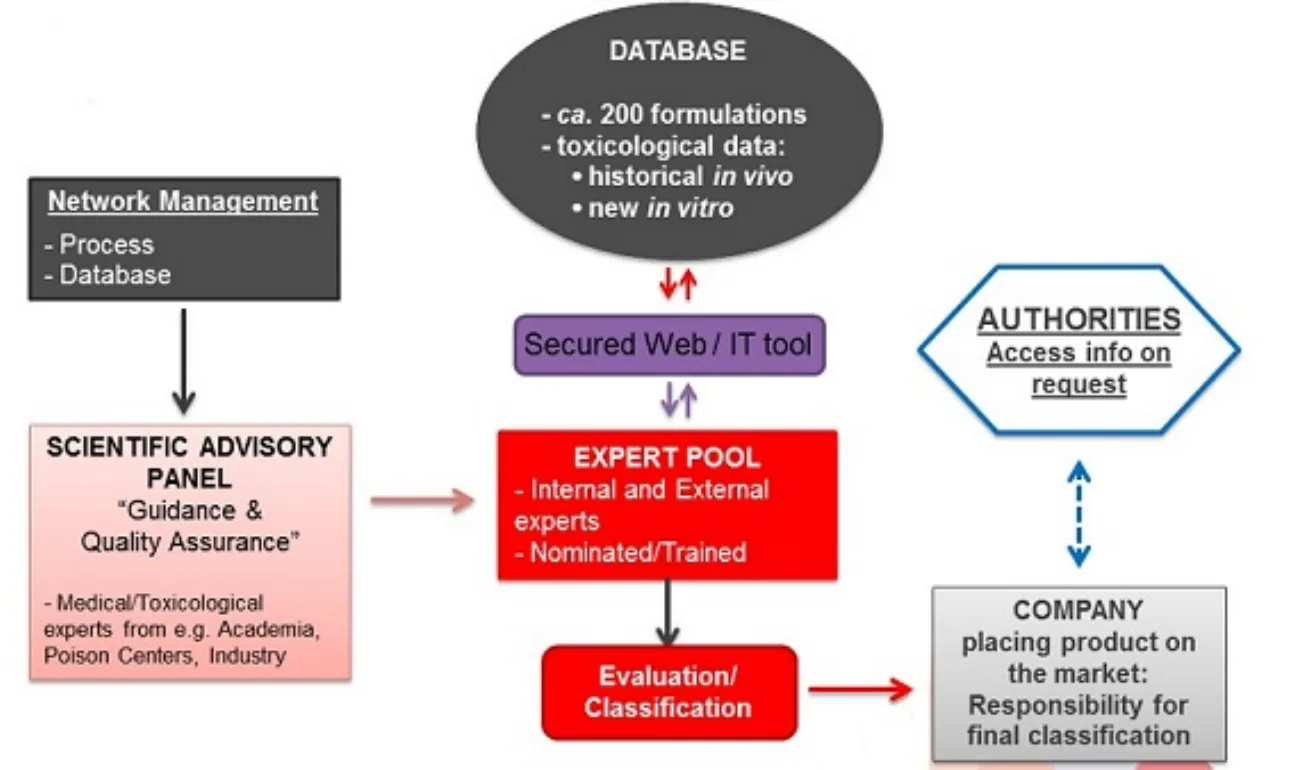
An overview of the DetNet structure is illustrated below:
-
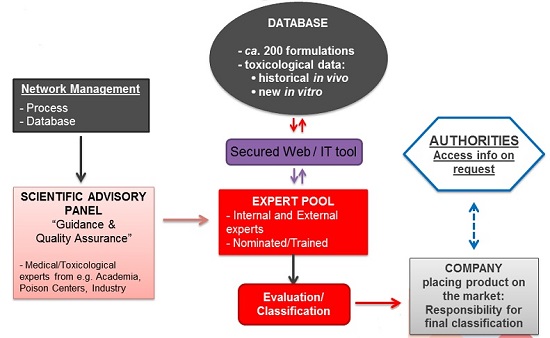
DetNet database
The cornerstone of DetNet is a database consisting of ca. 200 Reference Formulations.
-
Expert pool
This is the group of ‘classification experts’ (those that have been nominated by member companies, that meet the eligibility criteria and have followed the online training), i.e. individuals that will have access to the DetNet database and be able to derive classification on that basis. All experts need to sign a non-disclosure agreement.
The expert pool also includes ‘External Experts’ (proposed by A.I.S.E.) who are available for companies to use as needed in case the company does not have classification expertise in-house or a company expert needs a second opinion on a classification. If a company wants to use the services of an external expert, additional costs will apply. The actual final cost would depend on the contractual arrangements between the company requesting the service and the external expert.
-
Scientific Advisory Panel
The Scientific Advisory Panel (SAP) comprises recognised independent experts in the fields of toxicology, dermatology or ophthalmology who have been working with A.I.S.E. to ensure that DetNet is a robust and relevant means of correctly classify detergent and cleaning products.
SAP members act and decide independently and solely on the basis of scientific considerations, under full confidentiality.
The role of the SAP under DetNet is to provide advice to A.I.S.E. on the scientific approach, toxicological assessment, clinical experience and generation of reliable data (for classification purposes).
The list containing the names of SAP members is available here.
-
Access to authorities
Making classification data available to authorities, on request, is a legal requirement from CLP. At company level, classification records (including classification principles applied) should be kept for 10 years and made available to enforcement authorities in case of inspection. Further, A.I.S.E. will make available detailed mixture information to inspectors on request, namely the full composition of the Reference Formulation(s) used to derive classification and/or the corresponding full test reports.
How does it work ?
In practice, a company and its nominated expert(s) will go through the following steps:
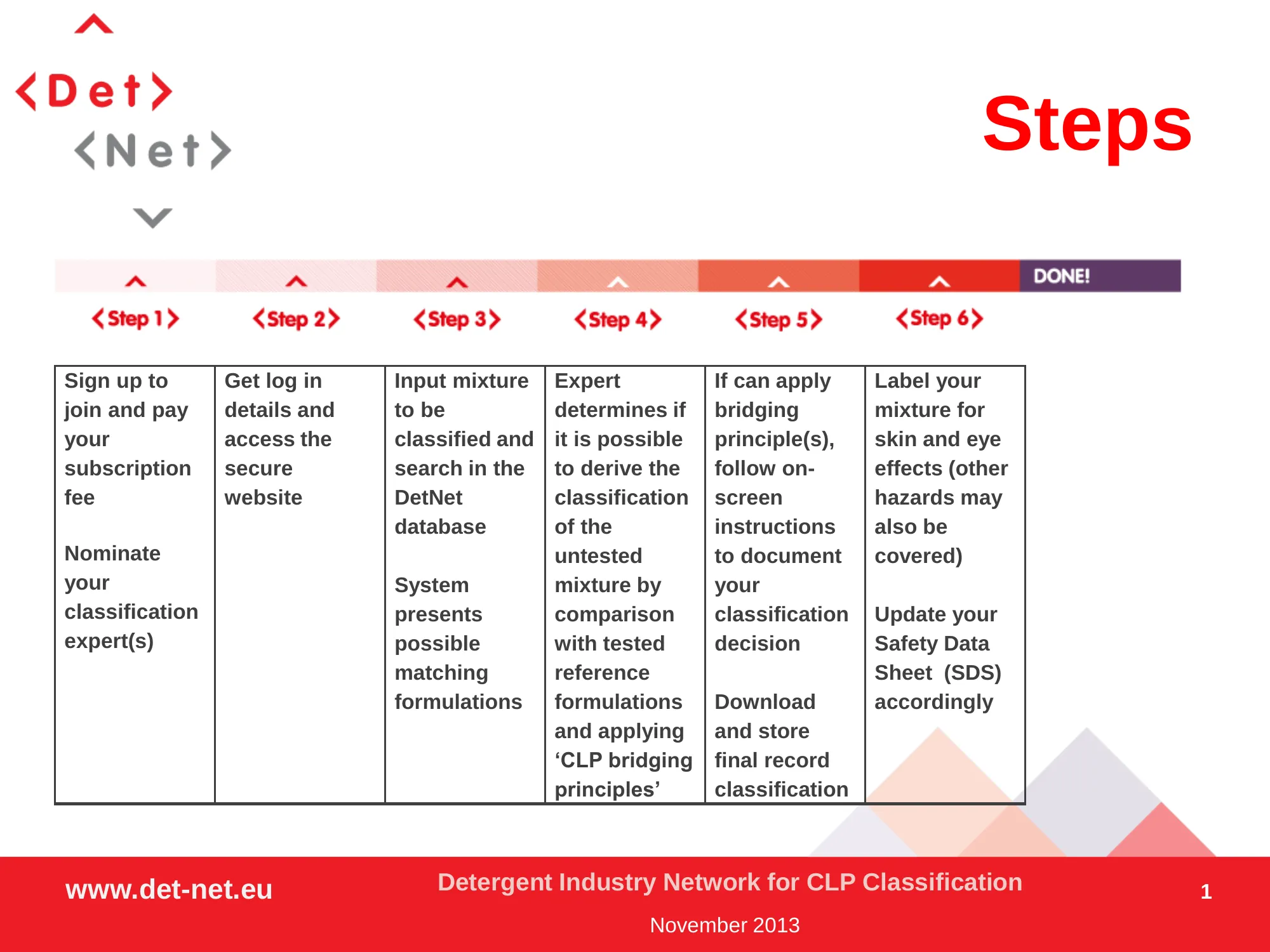
Download the Steps on-page overview
Experts
The DetNet database can be used by classification experts nominated by a company or by one of the external experts.
The role of an expert is to:
- Undertake training prior to the use of the DetNet database and demonstrate competency in application of the classification approach;
- Use the classification explanatory notes developed by A.I.S.E. to classify detergent and cleaning products for skin and eye irritation/corrosion;
- Compare the composition of the untested mixture to the reference formulations included in DetNet database and select the most appropriate ones for classification purposes, as applicable;
- Complete the agreed level of documentation to support classification decisions;
- Refer issues, inadequacies or problems with the guidance and gaps in the DetNet database to the DetNet manager (
This email address is being protected from spambots. You need JavaScript enabled to view it. ); - Discuss and explain classification decisions with national authorities when required - such issues are to be notified to DetNet manager (
This email address is being protected from spambots. You need JavaScript enabled to view it. ) which will provide support as necessary.
For more information on experts click here.
Scientific Advisory Panel
The Scientific Advisory Panel (SAP) comprises recognised independent experts in the fields of toxicology, dermatology or ophthalmology who have been working with A.I.S.E. to ensure that DetNet is a robust and relevant means of correctly classify detergent and cleaning products.
SAP members act and decide independently and solely on the basis of scientific considerations, under full confidentiality.
The role of the SAP under DetNet is to provide advice to A.I.S.E. on the scientific approach, toxicological assessment, clinical experience and generation of reliable data (for classification purposes).
DetNet database
The DetNet database includes ca. 200 formulations ('Reference Formulations'), covering a wide range of market relevant mixtures, each supported by skin and/or eye toxicological test data, either historical in vivo or data generated using in vitro methods.
The DetNet database provides a Reference Formulation Search Tool which allows identifying potential reference formulations for further evaluation. The reference formulations include laundry detergents (liquids and powders), hand dish wash detergents and all-purpose cleaners.
For each reference formulation the compositional details, the physical-chemical data and the toxicological summary of associated test data are available. Reference Formulation compositions are made available to experts from companies using a fixed format that provides sufficient detail to enable comparison of mixtures and application of bridging
principles, but still protects confidential business information of the data owner (e.g. no trade name of ingredient, use ‘perfume’ generically, etc.)
Toxicological data of Reference Formulations include in vitro data (generated using validated in vitro test methods, such as the EpiSkinTM Skin Irritation Test, and the Isolated Chicken Eye Test enhanced with histopathological assessment - for more information on the ICE test and histopathology, click here), as well as historical in vivo studies. All studies have been assessed for quality and reliability (Klimisch scoring) and study summaries are available for each test report.
An overview of the DetNet structure is illustrated below: