Welcome
CLP for end users
In light of new CLP changes, A.I.S.E. has prepared 2 brochures ('Read the label') for consumers and professional users with the purpose of triggering their awareness and explaining the changes in order to ensure the safe use of products. For more details click here.
See also the consumer information portal www.cleanright.eu.
How to classify a mixture under CLP
In practice, a company which needs to classify a mixture (e.g. for skin and eye effects) has the following options:
- Generate new in vitro test data on the complete mixture: this takes time and can be expensive if companies have a lot of mixtures to classify.
- Apply the bridging principles: this can only be done if test data exist for a number of ‘reference mixtures’ from which bridging is performed.
- Calculate the mixture’s classification using the additivity method. This is the easiest option, but it is also the most conservative one.
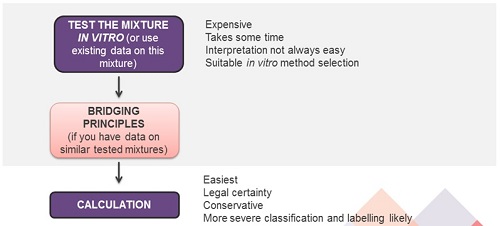
1. Generate in vitro data
In vitro test method must be suitable for the particular domain/formulation being tested. The selection of in vitro methods must be in line with Article 8(3) of CLP, i.e. be listed in the EU Test Methods Regulation or being a method internationally recognised. Some methods are validated only for certain effects/classification bands, this can be a limitation. In addition, not all laboratories are equipped to perform all tests. If the company has many mixtures to classify, this will take time (several weeks) and be expensive.
2. Apply bridging principles
Applying the bridging principles (see ECHA guidance on the application of the CLP criteria) can only be done if test data exist for similar mixtures (‘similar tested mixtures’) from which bridging is performed.
The CLP Regulation requires in Article 6(5) companies to use other available information on similar tested mixtures if no or inadequate test data on the mixture itself are available. The underlying concept encompasses the comparison (‘bridging’) of the scientific information and data pertinent to the assessment of the toxicological endpoint of interest (i.e., the skin or eye irritation/corrosion profile) of well-defined reference mixtures to newly developed detergent mixtures that are considered similar.
3. Classification by calculation
If data on the mixture itself or similar tested mixtures are not available, then the classification based on the ingredients in the mixture has to be done by calculation. This is well established, it can be automatic since calculation algorithms are provided in CLP. However, it is known that calculation rules are very conservative by design.
The classification by calculation would lead to the vast majority of daily use cleaning products (containing ≥ 3% Eye Cat 1 surfactant) being more severely classified and labelled with, for example, the corrosive pictogram (as shown below).

This classification result is due on one hand to the conservative CLP generic concentration limits for eye classification, and to the fact that many surfactants – the building blocks of detergents - are classified for serious eye damage ‘Eye Category 1’.
Under CLP, all detergents containing more than 3% of a surfactant classified ‘Eye Damage Category 1’ (H318) would be classified ‘Eye Damage Category 1’ based on the calculation rules.
Such a classification on daily use products such as hand dish wash or laundry detergents results in:
- The label not reflecting actual effects on man (based on human experience and historic in vivo data);
- Devaluing warning labels, with the risk that consumers/users can no longer recognise a truly hazardous product such as a drain cleaner or an oven cleaner containing sodium hydroxide from regular detergents;
- Confusing consumers/users;
- Leading to unsafe handling of products, both in the private and in the professional/industrial environment;
- Likely over-treatment in case of accidental exposure;
- Confusing poison centres.
Introduction
The Classification, Labelling and Packaging (CLP) Regulation (EC) No 1272/2008 governs the classification and labelling of chemical substances and mixtures, including household detergents and cleaning products.
It aligns EU legislation with the UN Globally Harmonised System (GHS) and has replaced the Dangerous Substances Directive (DSD) and Dangerous Preparations Directive (DPD).
CLP is a dynamic regulation, regularly updated through Adaptations to Technical Progress (ATP) to reflect scientific developments and revisions to the GHS. The most recent updates include:
- New hazard classes for endocrine disruptors (EDs) and persistent, mobile, and toxic substances (PMTs)
- Expanded harmonised classifications under Annex VI, with new substances added and existing entries revised
- Mandatory compliance dates for recent ATPs: e.g., the 23rd ATP (Regulation (EU) 2025/1222) will apply from 1 February 2027, with voluntary early adoption encouraged
The scope of CLP includes all substances and mixtures placed on the EU market, except those explicitly excluded under Article 1, such as:
- Radioactive substances,
- Non-isolated intermediates,
- Substances for scientific research not placed on the market,
- Finished products like cosmetics, medicinal products, and foodstuffs.
A key principle of CLP remains self-classification, unless a substance is listed in Annex VI with a harmonised classification. Classification must be based on all available data, including toxicological and ecotoxicological information.