Welcome
Classification by calculation
If data on the mixture itself or similar tested mixtures are not available, then the classification based on the ingredients in the mixture has to be done by calculation. This is well established, it can be automatic since calculation algorithms are provided in CLP. However, it is known that calculation rules are very conservative (by design).
The classification by calculation would lead to the vast majority of daily use cleaning products (containing ≥ 3% Eye Cat 1 surfactant) being more severely classified and labelled with, for example, the corrosive pictogram (as shown below).
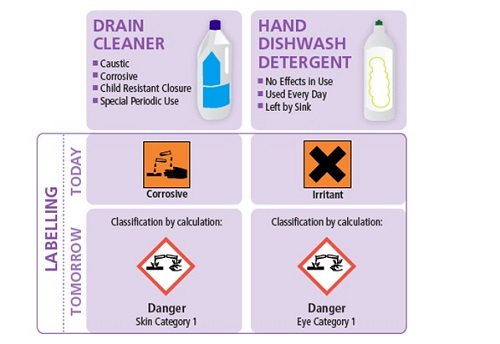
This change in labelling is due on one hand to the more conservative GHS/CLP generic concentration limits for eye classification, and, on the other hand, to the fact that many surfactants – the building blocks of detergents - are classified for serious eye damage ‘Eye Category 1’.
Today, the symbol applicable for ‘severe eye damage is the ‘Saint Andrew’s cross (as shown above). Under CLP, the ‘corrosive’ pictogram will have to be used on the label when a substance or a mixture is classified for serious eye damage.
Under CLP, all detergents containing more than 3% of a surfactant classified ‘Eye Damage Category 1’ (H318) would be classified ‘Eye Damage Category 1’ based on the calculation rules.
Such a classification on daily use products such as hand dish wash or laundry detergents would result in:
- The label not reflecting actual effects on man (based on human experience and historic in vivo data);
- Devaluing warning labels, with the risk that consumers/users can no longer recognise a truly hazardous product such as a drain cleaner or an oven cleaner containing sodium hydroxide from regular detergents;
- Confusing consumers/users;
- Leading to unsafe handling of products, both in the private and in the professional/industrial environment;
- Likely over-treatment in case of accidental exposure;
- Confusing poison centres.
Apply bridging principles
Applying the bridging principles (see ECHA guidance on the application of the CLP criteria) can only be done if test data exist for similar mixtures (‘similar tested mixtures’) from which bridging is performed.
The CLP Regulation requires in Article 6(5) companies to use other available information on similar tested mixtures if no or inadequate test data on the mixture itself are available. The underlying concept encompasses the comparison (‘bridging’) of the scientific information and data pertinent to the assessment of the toxicological endpoint of interest (i.e., the skin or eye irritation/corrosion profile) of well-defined reference mixtures to newly developed detergent mixtures that are considered similar.
Generate in vitro data
In vitro test method must be suitable for the particular domain/formulation being tested. The selection of in vitro methods must be in line with Article 8(3) of CLP, i.e. be listed in the EU Test Methods Regulation or being a method internationally recognised. Some methods are validated only for certain effects/classification bands, this can be a limitation. In addition, not all laboratories are equipped to perform all tests. If the company has many mixtures to classify, this will take time (several weeks) and be expensive.